Product details
English name: 3-KETO-N-VALERIC ACID METHYL ESTER;
3-KETOVALERIC ACID METHYL ESTER;
3-OXOPENTANOIC ACID METHYL ESTER;3-OXOVALERIC ACID METHYL ESTER;
METHYL 3-OXOPENTANOATE;METHYL 3-OXOVALERATE;METHYL PROPIONYLACETATE
MOP;PEM
PROPIONYLACETIC ACID METHYL ESTER;3-oxo-methylvalerate
Methy3-oxo-valerate;Methyl 3-oxo-n-valerate
Methyl beta-ketovalerate;methyl3-0xopentanoate
Valeric acid, 3-oxo-, methyl ester;Methylpropionlyacetate
Methyl propionylacetate Methyl 3-oxoval erate
METHYL 3-OXOVALERATE;Methyl-3-Oxo-Pentanoate
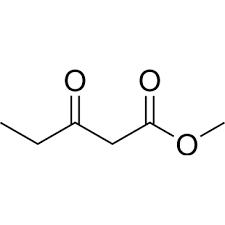
Molecular formula: C6H10O3
Molecular weight: 130.14
CAS NO:30414-53-0
Main properties:
Appearance: colorless transparent liquid
HP value: 4.3-5.6
Content: ≥98.0%
Moisture content: ≤0.20%
Packaging: 200Kg/barrel
Storage: cool and sealed
Methyl 3-oxopentanoate introduction
Methyl 3-oxopentanoate, also known as methyl ethyl acetoacetate, is a key compound widely utilized as a pharmaceutical intermediate. With its versatile chemical properties, it plays a crucial role in the synthesis of various pharmaceuticals, contributing significantly to advancements in drug discovery and development.
Chemical Structure and Properties
Methyl 3-oxopentanoate has the chemical formula C6H10O3 and is characterized by its ketone and ester functional groups. It appears as a clear, colorless liquid with a fruity aroma. These properties make it a valuable building block for the synthesis of complex molecules in pharmaceutical chemistry.
Chemical Formula: Methyl 3-oxopentanoate has the chemical formula C6H10O3. This formula indicates that the molecule contains six carbon atoms, ten hydrogen atoms, and three oxygen atoms.
Functional Groups: Methyl 3-oxopentanoate is characterized by two main functional groups: a ketone group (-C=O) and an ester group (-COOCH3). The ketone group is located at the third carbon atom in the pentanoate chain, giving rise to the "3-oxo" designation in its name.
Physical Appearance: Methyl 3-oxopentanoate appears as a clear, colorless liquid under standard conditions. It possesses a characteristic fruity aroma, which is often described as pleasant.
Solubility: This compound is soluble in polar solvents such as water, ethanol, and acetone. Its solubility in non-polar solvents may vary depending on the specific solvent and temperature conditions.
Boiling Point: Methyl 3-oxopentanoate has a relatively high boiling point, typically ranging between 140-150°C. The exact boiling point may vary depending on factors such as purity and atmospheric pressure.
Density: The density of Methyl 3-oxopentanoate is approximately 1.06 g/cm³ at room temperature. This value may also vary slightly depending on factors such as temperature and impurities.
Reactivity: Due to the presence of functional groups, Methyl 3-oxopentanoate exhibits reactivity towards various chemical reactions. It can undergo nucleophilic addition, ester hydrolysis, and condensation reactions, making it a versatile building block in organic synthesis.
Stability: Methyl 3-oxopentanoate is relatively stable under normal storage and handling conditions. However, it may undergo degradation upon exposure to heat, light, or strong acids or bases.
Applications: The unique chemical structure and properties of Methyl 3-oxopentanoate make it a valuable building block for the synthesis of complex molecules in pharmaceutical chemistry. It serves as a precursor in the production of various pharmaceuticals and pharmaceutical intermediates, contributing to drug discovery and development efforts.
Methyl 3-oxopentanoate is characterized by its chemical formula C6H10O3, ketone and ester functional groups, clear and colorless liquid appearance, and fruity aroma. These properties, along with its solubility, boiling point, density, reactivity, and stability, make it a versatile compound with significant applications in pharmaceutical chemistry as a valuable building block for the synthesis of complex molecules.
Synthesis
Methyl 3-oxopentanoate can be synthesized through the reaction of ethyl acetoacetate with methanol in the presence of acid catalysts. The process involves esterification followed by decarboxylation, yielding the desired product in good yields. Alternatively, it can be obtained from other synthetic routes or commercially available sources.
Esterification
The initial step involves the reaction of ethyl acetoacetate with methanol. This reaction is typically carried out in the presence of an acid catalyst, such as sulfuric acid or p-toluenesulfonic acid.
Ethyl acetoacetate (also known as ethyl 3-oxobutanoate) contains a reactive keto group and an ester group. In the presence of methanol and an acid catalyst, the ester group undergoes nucleophilic substitution by methanol, leading to the formation of methyl ester.
The acid catalyst facilitates the protonation of the carbonyl oxygen, increasing its electrophilicity and promoting nucleophilic attack by methanol. This results in the formation of a tetrahedral intermediate, which subsequently undergoes dehydration to yield Methyl 3-oxopentanoate.
Decarboxylation
After esterification, the obtained methyl ester undergoes decarboxylation to yield Methyl 3-oxopentanoate.
Decarboxylation is a process in which a carboxylic acid group is removed from a compound, resulting in the loss of carbon dioxide (CO2) and the formation of a ketone group.
In the case of Methyl 3-oxopentanoate synthesis, the ethyl acetoacetate undergoes decarboxylation upon heating or under acidic conditions. This leads to the removal of the carboxylic acid group (-COOH) from the ester, resulting in the formation of the desired product, Methyl 3-oxopentanoate.
Yield and Purification
The esterification and decarboxylation reactions typically yield Methyl 3-oxopentanoate in good yields.
After synthesis, the product may undergo purification steps, such as distillation or recrystallization, to remove any impurities and obtain the desired compound in high purity.
Alternative Synthesis Routes
Apart from the described route, Methyl 3-oxopentanoate can also be obtained from alternative synthetic routes or commercially available sources.
These alternative routes may involve different starting materials or reaction conditions but ultimately result in the formation of Methyl 3-oxopentanoate as the desired product.
The synthesis of Methyl 3-oxopentanoate involves esterification of ethyl acetoacetate with methanol followed by decarboxylation, resulting in the formation of the desired compound. This process can be carried out efficiently with good yields, contributing to its widespread availability for various applications in the pharmaceutical and chemical industries.
Applications in Pharmaceutical Intermediates
Drug Synthesis
Methyl 3-oxopentanoate serves as a versatile intermediate in the synthesis of a wide range of pharmaceuticals. It undergoes various chemical transformations, including condensation, alkylation, and cyclization reactions, to yield structurally diverse drug molecules.
Condensation Reactions:
In drug synthesis, Methyl 3-oxopentanoate participates in condensation reactions, wherein it reacts with other appropriate reagents to form key intermediates or final drug products. Condensation reactions involve the combination of two or more molecules, often leading to the formation of a larger molecule with the elimination of a small molecule such as water or alcohol. Methyl 3-oxopentanoate's ketone and ester functional groups make it particularly amenable to condensation reactions, allowing for the creation of complex molecular structures essential for drug activity.
Alkylation Reactions:
Another crucial aspect of drug synthesis involving Methyl 3-oxopentanoate is its involvement in alkylation reactions. Alkylation involves the addition of alkyl groups to a molecule, often resulting in the introduction of specific functional groups or modifications that enhance the drug's pharmacological properties. Methyl 3-oxopentanoate serves as a versatile substrate in alkylation reactions, enabling the introduction of alkyl groups at specific positions within the molecular scaffold, thereby diversifying the chemical space accessible for drug design.
Cyclization Reactions:
Cyclization reactions represent yet another essential avenue for utilizing Methyl 3-oxopentanoate in drug synthesis. Cyclization involves the formation of cyclic structures within a molecule, which can significantly influence the molecule's bioactivity and pharmacokinetic properties. Methyl 3-oxopentanoate's ability to undergo cyclization reactions allows for the construction of cyclic motifs within drug molecules, imparting desirable structural features and enhancing their potential therapeutic efficacy.
Structural Diversity and Drug Design:
The versatility of Methyl 3-oxopentanoate in participating in various chemical transformations affords drug designers the flexibility to tailor the molecular structures of pharmaceutical compounds to achieve desired pharmacological effects. By incorporating Methyl 3-oxopentanoate as a key intermediate, researchers can access a diverse array of structural motifs, thereby expanding the chemical space available for drug design and optimization.
Methyl 3-oxopentanoate serves as an indispensable intermediate in the synthesis of pharmaceuticals, facilitating condensation, alkylation, and cyclization reactions to yield structurally diverse drug molecules. Its role in drug synthesis underscores its significance as a key building block in the development of innovative therapeutic agents. By harnessing the chemical versatility of Methyl 3-oxopentanoate, researchers continue to advance the frontier of drug discovery and development, striving to address unmet medical needs and improve patient outcomes.
Active Pharmaceutical Ingredients (APIs)
This compound is utilized in the production of active pharmaceutical ingredients (APIs) for medications targeting different therapeutic areas. It serves as a key precursor in the synthesis of analgesics, antipyretics, antivirals, and other drugs.
Synthesis of Analgesics and Antipyretics
One prominent application of Methyl 3-oxopentanoate lies in the synthesis of analgesics and antipyretics, medications crucial for alleviating pain and reducing fever, respectively. Through strategic chemical transformations, Methyl 3-oxopentanoate serves as the foundation for the synthesis of active ingredients such as nonsteroidal anti-inflammatory drugs (NSAIDs) and acetaminophen, which are widely used to manage pain and fever associated with various medical conditions.
Synthesis of Antivirals
Methyl 3-oxopentanoate also finds extensive use in the production of antiviral medications, which play a vital role in combating viral infections. By serving as a key intermediate in the synthesis of antiviral agents, Methyl 3-oxopentanoate contributes to the development of pharmaceuticals aimed at treating infections caused by viruses such as influenza, herpes simplex virus, and human immunodeficiency virus (HIV). These medications are instrumental in preventing viral replication and curtailing the progression of viral diseases.
Synthesis of Other Drugs
In addition to analgesics, antipyretics, and antivirals, Methyl 3-oxopentanoate serves as a versatile precursor in the synthesis of various other drugs targeting different therapeutic indications. Its chemical versatility allows for the creation of structurally diverse molecules with distinct pharmacological properties, including but not limited to anti-inflammatory agents, cardiovascular drugs, and central nervous system (CNS) medications. By serving as a key building block in API synthesis, Methyl 3-oxopentanoate facilitates the production of pharmaceuticals aimed at addressing a myriad of medical conditions and improving patient outcomes.
Methyl 3-oxopentanoate plays a pivotal role in the synthesis of active pharmaceutical ingredients (APIs) for medications targeting diverse therapeutic areas. Its utilization as a key precursor in the synthesis of analgesics, antipyretics, antivirals, and other drugs underscores its significance in pharmaceutical chemistry. By enabling the production of API molecules with distinct pharmacological properties, Methyl 3-oxopentanoate contributes to the development of innovative pharmaceuticals aimed at addressing unmet medical needs and improving patient care.
Heterocyclic Compounds
Methyl 3-oxopentanoate is commonly employed in the synthesis of heterocyclic compounds such as pyrazoles, pyrimidines, and pyrroles. These compounds exhibit diverse biological activities and are valuable scaffolds for drug design and development.
Synthesis of Pyrazoles
Pyrazoles are a class of heterocyclic compounds characterized by a five-membered ring containing two nitrogen atoms. Methyl 3-oxopentanoate is commonly employed in the synthesis of pyrazoles through condensation reactions with hydrazine derivatives or amidines. These reactions lead to the formation of pyrazole rings, which serve as key structural motifs in various pharmaceuticals, including nonsteroidal anti-inflammatory drugs (NSAIDs) and pesticides.
Synthesis of Pyrimidines
Pyrimidines represent another class of heterocyclic compounds featuring a six-membered ring containing two nitrogen atoms. Methyl 3-oxopentanoate participates in the synthesis of pyrimidines through multistep reactions involving condensation, cyclization, and functional group transformations. Pyrimidines are integral components of nucleic acids (DNA and RNA) and serve as the core structure in many pharmacologically active compounds, including anticancer and antiviral drugs.
Synthesis of Pyrroles
Pyrroles are heterocyclic compounds characterized by a five-membered ring containing one nitrogen atom. Methyl 3-oxopentanoate is utilized in the synthesis of pyrroles through the Knorr or Paal-Knorr reactions, involving the condensation of Methyl 3-oxopentanoate with primary amines and subsequent cyclization. Pyrroles are essential components in various biologically active molecules, including heme in hemoglobin and myoglobin, as well as certain pharmaceuticals with antibacterial and antifungal properties.
Biological Activities and Drug Development
Heterocyclic compounds synthesized using Methyl 3-oxopentanoate as a precursor exhibit diverse biological activities, ranging from antimicrobial and anti-inflammatory to anticancer and antiviral properties. The structural diversity afforded by heterocyclic scaffolds enables the design of molecules with specific pharmacological profiles, making them attractive targets for drug development. Moreover, the incorporation of heterocyclic motifs into drug molecules enhances their bioavailability, metabolic stability, and target specificity, thereby improving their therapeutic efficacy.
Methyl 3-oxopentanoate plays a pivotal role in the synthesis of heterocyclic compounds such as pyrazoles, pyrimidines, and pyrroles. These compounds serve as valuable scaffolds for drug design and development, exhibiting diverse biological activities and pharmacological properties. By enabling the synthesis of heterocyclic molecules, Methyl 3-oxopentanoate contributes to the expansion of the chemical space available for drug discovery, ultimately leading to the development of novel therapeutic agents for various medical conditions.
Beta-Keto Esters
As a beta-keto ester, methyl 3-oxopentanoate is particularly valuable in the synthesis of beta-keto ester derivatives. These compounds are important intermediates for the preparation of β-lactams, β-amino acids, and other biologically active molecules.
Synthesis of Beta-Keto Esters
Beta-keto esters are compounds characterized by the presence of a carbonyl group (keto) and an ester group adjacent to each other on the same carbon atom (beta position) within the molecular structure. Methyl 3-oxopentanoate serves as a valuable precursor in the synthesis of beta-keto esters through a variety of synthetic routes. One common approach involves the condensation of Methyl 3-oxopentanoate with suitable carbonyl compounds, followed by subsequent keto-enol tautomerization to yield the desired beta-keto ester.
Importance of Beta-Keto Esters
Beta-keto esters and their derivatives play a pivotal role in organic synthesis due to their versatility and reactivity. These compounds serve as key intermediates in the construction of complex molecular scaffolds, enabling the synthesis of diverse classes of biologically active molecules. Beta-keto esters undergo a variety of transformations, including decarboxylation, reduction, and nucleophilic addition reactions, which allows for the facile introduction of functional groups and structural modifications.
Synthesis of β-Lactams
Beta-keto esters serve as precursors in the synthesis of β-lactams, a class of heterocyclic compounds characterized by a four-membered lactam ring. β-Lactams are integral components of many antibiotics, including penicillins, cephalosporins, and carbapenems, which exhibit potent antimicrobial activity against bacterial pathogens. Methyl 3-oxopentanoate-derived beta-keto esters undergo cyclization and subsequent ring expansion reactions to form the β-lactam ring system, providing access to these important antibiotic agents.
Synthesis of β-Amino Acids
Additionally, beta-keto esters are utilized in the synthesis of β-amino acids, which represent a valuable class of compounds with diverse biological activities. β-Amino acids serve as building blocks for peptide and protein synthesis, as well as pharmaceutical agents targeting various therapeutic indications. Methyl 3-oxopentanoate-derived beta-keto esters can be transformed into β-amino acids through selective reduction and functional group manipulation reactions, providing access to these important molecular entities.
Methyl 3-oxopentanoate plays a crucial role in the synthesis of beta-keto esters, which serve as versatile intermediates for the preparation of β-lactams, β-amino acids, and other biologically active molecules. Through strategic synthetic transformations, Methyl 3-oxopentanoate-derived beta-keto esters enable the construction of complex molecular scaffolds essential for drug discovery and development. The utilization of beta-keto esters in organic synthesis underscores their importance as valuable tools for accessing diverse chemical space and expanding the repertoire of biologically relevant compounds.
Prodrugs
Methyl 3-oxopentanoate can be used as a prodrug moiety to enhance the pharmacokinetic properties of certain drugs. By masking functional groups, it facilitates better drug delivery, bioavailability, and metabolic stability.
Prodrug Design
Prodrugs are inactive or less active derivatives of pharmacologically active compounds that undergo enzymatic or chemical transformation in vivo to release the active drug. In the case of Methyl 3-oxopentanoate, it is utilized as a prodrug moiety by chemically conjugating it to the parent drug molecule. This conjugation temporarily masks or alters certain functional groups, rendering the prodrug inactive or less active until it undergoes metabolic or chemical transformation to release the active drug.
Enhanced Drug Delivery
Methyl 3-oxopentanoate as a prodrug moiety can enhance drug delivery by altering the physicochemical properties of the parent drug. By masking polar or charged functional groups, the prodrug can exhibit improved lipophilicity, which facilitates better absorption across biological membranes. This enhanced membrane permeability allows for increased drug distribution to target tissues or organs, thereby improving the efficacy of the therapeutic agent.
Improved Bioavailability
Bioavailability refers to the fraction of an administered dose of drug that reaches systemic circulation in an unchanged form and is available to exert its pharmacological effect. Methyl 3-oxopentanoate prodrugs can enhance bioavailability by overcoming various barriers to drug absorption, metabolism, and elimination. By optimizing the physicochemical properties of the parent drug, such as solubility and membrane permeability, the prodrug can increase the fraction of drug absorbed into systemic circulation, thereby enhancing overall bioavailability.
Enhanced Metabolic Stability
Metabolic stability refers to the ability of a drug molecule to resist enzymatic degradation and maintain its pharmacological activity in vivo. Methyl 3-oxopentanoate prodrugs can improve metabolic stability by protecting susceptible functional groups from enzymatic degradation. The conjugation of Methyl 3-oxopentanoate to the parent drug molecule can hinder or delay metabolic processes, thereby prolonging the systemic exposure of the active drug and enhancing its therapeutic effect.
Methyl 3-oxopentanoate serves as a valuable prodrug moiety in pharmaceutical science, offering a strategy to enhance the pharmacokinetic properties of certain drugs. By masking functional groups, Methyl 3-oxopentanoate prodrugs facilitate better drug delivery, improved bioavailability, and enhanced metabolic stability. This innovative approach to prodrug design holds promise for optimizing the therapeutic efficacy and safety of pharmaceutical agents, ultimately benefiting patients by improving treatment outcomes.
Conclusion
Methyl 3-oxopentanoate plays a vital role as a pharmaceutical intermediate, offering versatility and synthetic utility in drug synthesis. Its importance extends across various stages of drug development, from lead optimization to API production. With ongoing advancements in medicinal chemistry and pharmaceutical science, the significance of methyl 3-oxopentanoate as a key building block is expected to continue growing.